When your liver starts sending mixed signals, it’s not always easy to tell what’s really going on. Imagine having symptoms that look like one disease, but the blood tests point to another - and the biopsy shows a third. This isn’t science fiction. It’s real life for people with autoimmune overlap syndromes, where two or more autoimmune liver diseases show up at the same time. The most common mix involves Primary Biliary Cholangitis (PBC), Primary Sclerosing Cholangitis (PSC), and Autoimmune Hepatitis (AIH). But here’s the catch: not all combinations are real. Some are myths. Others are dangerously easy to miss.
What Exactly Is an Autoimmune Overlap Syndrome?
An autoimmune overlap syndrome happens when a person has clear signs of more than one autoimmune liver disease at once. It’s not just having two conditions side by side - it’s having them tangled together in the same liver. The most well-documented overlap is between AIH and PBC. About 1 to 3% of people with PBC also show features of AIH. In people diagnosed with AIH, up to 7% turn out to have PBC-like traits. That might sound rare, but when you’re dealing with chronic liver disease, even small percentages mean thousands of people. The tricky part? These aren’t separate diseases living in the same body. They’re overlapping patterns - like two different fingerprints smudged together on the same glass. The liver doesn’t care about labels. It just reacts. And that reaction can look like a mix of everything: inflammation, bile duct damage, scarring, and abnormal antibodies.Breaking Down the Three Diseases
To understand overlap, you need to know the players. PBC (Primary Biliary Cholangitis) attacks the small bile ducts inside the liver. It’s mostly seen in women over 40. The telltale sign? A sharp rise in alkaline phosphatase (ALP) and gamma-glutamyl transferase (γ-GT). Over 90% of PBC patients have anti-mitochondrial antibodies (AMA) in their blood - a marker so specific it’s almost a fingerprint. Left untreated, PBC slowly destroys bile flow, leading to scarring, cirrhosis, and eventually liver failure. AIH (Autoimmune Hepatitis) goes after the liver cells themselves. It causes inflammation right in the liver tissue. The key markers? High ALT and AST (transaminases), elevated IgG, and autoantibodies like ANA (antinuclear antibodies) or SMA (smooth muscle antibodies). Unlike PBC, AIH doesn’t always show up on blood tests with bile duct markers. Instead, it shows up as liver cell damage. Without treatment, AIH can lead to cirrhosis in less than 10 years. PSC (Primary Sclerosing Cholangitis) is the odd one out. It affects the larger bile ducts - both inside and outside the liver. It’s often linked to inflammatory bowel disease, especially ulcerative colitis. Like PBC, it raises ALP and γ-GT. But unlike PBC, PSC patients rarely have AMA. And unlike AIH, there’s no clear antibody marker. Diagnosis often requires an MRI scan called MRCP to see the bile ducts. PSC is progressive, unpredictable, and carries a higher risk of bile duct cancer.The Real Overlap: AIH and PBC
The only overlap syndrome with enough evidence to be taken seriously is AIH-PBC. It’s not a new disease. It’s a hybrid. A patient might have the high ALP and AMA of PBC, but also the elevated transaminases and IgG of AIH. Their biopsy might show both bile duct damage and interface hepatitis - the classic sign of AIH. Doctors use a loose set of criteria to diagnose it: at least two features from PBC and two from AIH. For PBC, that means elevated ALP, positive AMA (or sp100/gp210 antibodies if AMA is negative), and bile duct damage on biopsy. For AIH, it’s elevated IgG, positive ANA or SMA, and interface hepatitis on biopsy. One study of 130 PBC patients found that 9% met these combined criteria. Another looked at 199 patients with either AIH or PBC and found 8% had overlap features. These aren’t outliers. They’re signals.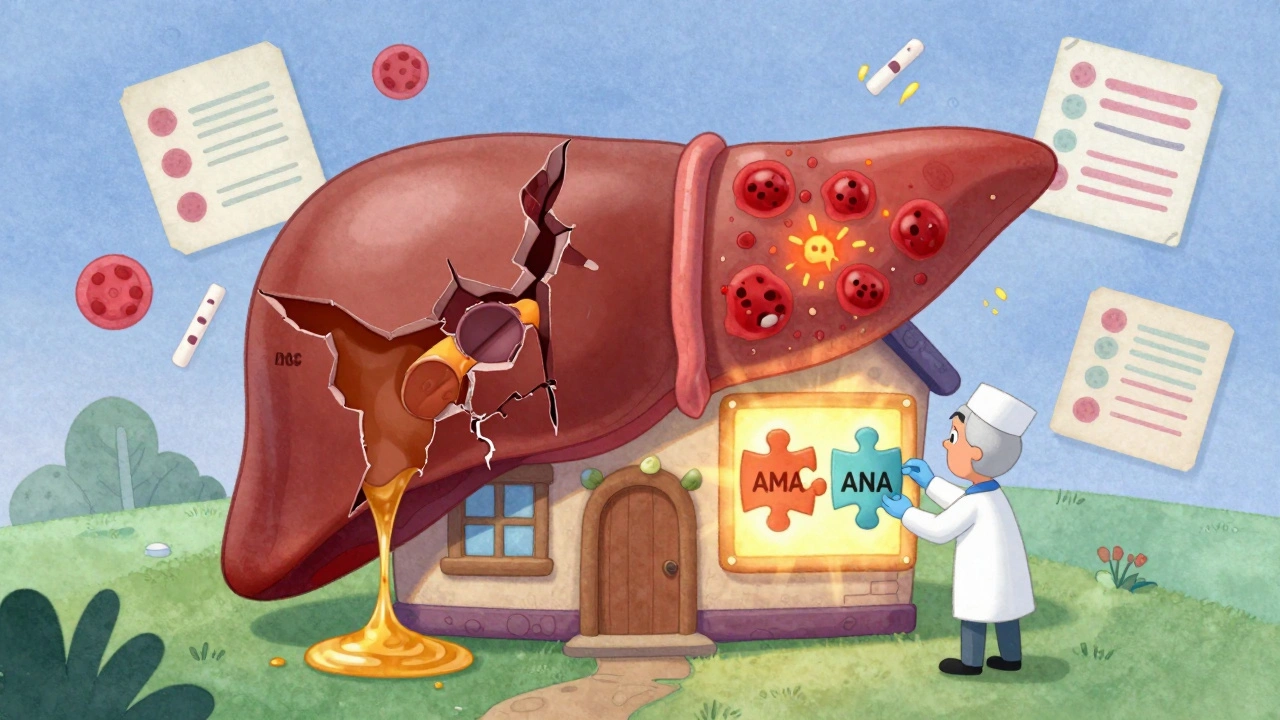
Why PBC-PSC Overlap Is Likely a Myth
You might read about cases where someone has features of both PBC and PSC. But here’s the truth: experts don’t believe a true PBC-PSC overlap exists. Why? Because their patterns don’t mix. PBC is defined by AMA and small bile duct destruction. PSC is defined by large duct scarring and no AMA. If you have AMA, you likely have PBC. If you have large duct strictures on an MRI and no AMA, you likely have PSC. The two don’t coexist in the same liver in a meaningful way. Some case reports claim to see both, but these are likely misdiagnoses - maybe the patient has PBC with secondary bile duct changes, or PSC with a false-positive AMA. The World Journal of Gastroenterology and other major reviews have concluded: there’s no clear evidence for PBC-PSC overlap. It’s a red flag when someone says they have it. Double-check the tests.How Doctors Diagnose Overlap - And Why It’s So Hard
Diagnosing overlap isn’t a single test. It’s a puzzle. First, blood work. You need to check for ALP, AST, ALT, IgG, IgM, AMA, ANA, SMA, and sometimes sp100/gp210. If ALP is high and AMA is positive, that’s PBC. If ALT and AST are sky-high and IgG is up with ANA, that’s AIH. But if both sets are elevated? That’s the red flag. Then comes the biopsy. It’s not always required for PBC, but if there’s any doubt - especially if transaminases are high - you need it. A biopsy can show bile duct loss (PBC) and interface hepatitis (AIH) in the same sample. That’s the gold standard. The problem? Many doctors aren’t trained to look for this. In community clinics, misdiagnosis rates hit 15-20%. A patient gets labeled with PBC, put on ursodeoxycholic acid (UDCA), and feels worse. Why? Because they also have AIH - and UDCA doesn’t touch inflammation.Treatment: One Drug Isn’t Enough
PBC? UDCA is the go-to. AIH? Steroids and azathioprine. Simple, right? Not in overlap. About 30-40% of AIH-PBC patients don’t respond well to UDCA alone. Their liver enzymes stay high. Their inflammation doesn’t calm down. That’s because UDCA treats bile flow - not immune attack. These patients need both. UDCA to protect the bile ducts, plus immunosuppressants like prednisone or azathioprine to quiet the immune system. Some patients need both from day one. Others start with UDCA and add steroids only if their transaminases don’t drop after 6 months. Treatment isn’t one-size-fits-all. It’s tailored. If IgG is sky-high and interface hepatitis is clear on biopsy, start steroids early. If AMA is strong and transaminases are only mildly elevated, try UDCA first. Watch closely. Adjust as you go.


ruiqing Jane
December 3, 2025 AT 08:58It’s wild how often overlap syndromes get mislabeled as one disease when they’re clearly a hybrid. I’ve seen patients on UDCA for years with persistent ALT spikes-no one thought to check for AIH until the biopsy showed interface hepatitis. That’s not just a missed diagnosis; it’s a systemic blind spot in hepatology training. We need to train residents to look for the *combination*, not just the textbook case.
And yes, AMA-negative PBC with sp100/gp210? That’s real. I’ve had two patients in my clinic with that exact profile. One was misdiagnosed as AIH for three years. The other? Treated as PBC until her IgG hit 2x normal. Biopsy saved both.
Stop assuming one marker = one disease. The liver doesn’t care about your algorithm.
Fern Marder
December 3, 2025 AT 09:35So… PBC-PSC overlap is a myth?? 😳 I just read a blog post last week that was like ‘OMG I have both!!’ and now I’m questioning everything. Like… who’s lying? The patient? The doctor? The internet?? 🤯
Allan maniero
December 4, 2025 AT 21:52I’ve been managing liver patients for over 18 years, and I’ve seen this pattern play out too many times. The real tragedy isn’t the misdiagnosis-it’s the delay in treatment. I had a patient in 2019 who was told she had ‘mild PBC’ for four years. Her ALT was always just a little elevated, so no one pushed for a biopsy. By the time we did, she had stage 3 fibrosis and clear interface hepatitis. She needed steroids, but we lost precious time.
It’s not that doctors are negligent. It’s that the system doesn’t reward digging deeper. Insurance won’t pay for a biopsy unless the numbers are screaming. But in overlap syndromes, the numbers whisper-and too many of us are too tired to lean in.
And yes, the PBC-PSC overlap myth? Absolutely. I’ve reviewed 12 cases that claimed it. Nine were AMA-positive PBC with secondary duct changes. Two were PSC with a false AMA due to recent infection. One was just a misread MRI. We need better education, not more case reports.
Anthony Breakspear
December 6, 2025 AT 09:18Let’s be real-this isn’t just medicine. It’s detective work with a liver biopsy as the smoking gun. 🕵️♂️🧬
I’ve seen people get labeled PBC, put on UDCA, and then get sicker. Why? Because their immune system is still attacking hepatocytes. UDCA doesn’t touch that. It’s like putting a bandaid on a bullet wound and calling it done.
And if your IgG is sky-high and your biopsy shows interface hepatitis? Stop playing it safe. Start steroids. Don’t wait six months. That’s not caution-that’s negligence dressed up as ‘watchful waiting.’
Also, PBC-PSC overlap? Nah. That’s like saying you have a cat and a dog that’s also a parrot. Maybe you’ve got a weird pet, but it’s not a hybrid species. Same logic here. Check your AMA. Check your MRCP. Don’t make up diseases because the chart looks messy.
Zoe Bray
December 7, 2025 AT 05:51It is imperative to underscore that the diagnostic criteria for autoimmune overlap syndromes, particularly AIH-PBC, must be applied with strict adherence to the International Autoimmune Hepatitis Group (IAIHG) scoring system, augmented by the PBC-specific criteria for AMA, sp100, and gp210 serology, alongside histopathological confirmation of interface hepatitis and bile duct destruction. Failure to employ a multimodal diagnostic approach results in therapeutic suboptimization and accelerated fibrogenesis.
Furthermore, the absence of formalized diagnostic criteria by AASLD and EASL remains a significant impediment to standardization across tertiary care centers. Until consensus guidelines are promulgated, clinicians must rely on expert opinion, which introduces significant inter-observer variability.
Therapeutic protocols must be individualized, with immunosuppression initiated concurrently with UDCA in patients demonstrating both biochemical and histological features of AIH, irrespective of initial diagnostic label. Delayed intervention correlates directly with increased transplant candidacy.
Girish Padia
December 8, 2025 AT 18:09People waste so much time on this. You got sick? Go get a second opinion. Don’t waste your life reading blogs. I saw a guy in Delhi who spent 5 years chasing ‘overlap’-turned out he had alcohol-related liver damage and was too proud to admit it. Stop overcomplicating. If your ALP is up, stop drinking. If your ALT is up, stop eating junk. Done.
Also, AMA? Who cares. Just treat the liver. Not the antibodies.
Saket Modi
December 9, 2025 AT 12:41so like... i have pbc and i think i have aiH too? 😭
my doctor just gave me udca and said 'it's fine'...
but i'm so tired all the time and my joints hurt
and i just wanna know if i'm crazy or if this is real
😭😭😭
Chris Wallace
December 11, 2025 AT 08:28Reading this reminded me of a patient I saw last year. She was 52, asymptomatic, diagnosed with PBC after routine labs showed elevated ALP. AMA positive. Everything looked textbook. But her ALT was 85-mildly elevated, so we didn’t push further. Six months later, she came back with fatigue and joint pain. Her IgG was through the roof. Biopsy showed clear interface hepatitis. She’d had AIH-PBC overlap the whole time.
We started her on UDCA and azathioprine. Within three months, her transaminases dropped into normal range. She cried when she told me she’d slept through the night for the first time in two years.
It’s not just about the numbers. It’s about listening to the person behind them. Sometimes the symptoms are the only clue the liver gives you before it shuts down.
And yeah-PBC-PSC overlap? Still a myth. I’ve seen too many mislabeled MRIs. Don’t trust a radiologist’s one-line report if the serology doesn’t add up.
william tao
December 11, 2025 AT 16:32Let’s be honest-this entire field is a mess. Overlap syndromes? More like ‘we didn’t know what we were looking at, so we made up a label.’
Biopsies are invasive. Antibodies are expensive. Doctors are overworked. And patients? They’re just tired of being told ‘it’s probably nothing.’
Meanwhile, pharmaceutical companies are quietly funding ‘overlap’ research because combination therapies = more prescriptions. Steroids + UDCA? That’s two drugs, two co-pays, two follow-ups. Profit.
And don’t get me started on the ‘sp100/gp210’ hype. It’s not magic. It’s just another antibody that costs $300 and changes nothing if you don’t treat the inflammation.
We’re treating antibodies like they’re the disease. They’re not. They’re signs. The disease is the liver dying. And we’re still arguing over the label instead of saving lives.